Why Quantum Mechanic Must Be Used to Describe Electrons
The quantum mechanical model of the atom. Start studying Quantum Mechanics and Electron Configuration.

Quantum Mechanical Model Overview History Expii
We know that all molecules are made of atoms that in turn contain nuclei and electrons.

. We are made of matter thus particles. If the quantum wave natures of the individual bits of matter can be aligned into a coherent state then quantum effects will become evident on the macroscopic scale. Semiempirical quantum mechanical SM methods offer further approximation of molecular models supplementing the MM calculation for a deeper insight into the electron distribution described by molecular orbitals.
Therefor no electrons can move because of the exclusion principle there can never be to electrons at the same exact state. Faster-moving particles those moving at a few percentages of the speed of light or faster are noticeably affected by Special Relativity. When a conducting material is cooled enough its conduction electrons spread out into large-scale.
2 maximum of electrons in each energy level corresponds to the of elements in each period of the periodic table 2 8818 3 explained the line spectrum of hydrogen. Molecular orbitals are calculated in SM methods on the different levels of approximation while including only valence electrons. Introduction to the quantum mechanical model of the atom.
Failure of Bohr model. The best description we have of the nature of the particles that make up matter is described by quantum mechanics. And all electrons in singly occupied orbitals must have the same spin is a statement of.
1 gives a reasonable explanation for Mendeleevs periodic law. The angular momentum number is the shape of the orbital holding the electron. Specifically this probability is.
Quantum mechanics wave mechanics Does not allow us to specify exact location of electrons we can predict high probability of finding an electron Use the term atomic orbital instead of orbit to describe the electrons position within the atom Quantum Mechanics. Classical models using Newtonian forces and electromagnetism can explain certain motions of electrons. Assign quantum numbers to electrons noting their physical meaning Key Takeaways Key Points To completely describe an electron in an atom four quantum numbers are needed.
Four quantum numbers are used to describe electrons. Their structures energies and other properties have only been successfully described within the framework of quantum mechanics. This is the currently selected item.
The way that atoms absorb and re-emit light is described by quantum theory. Electron spin and the Stern-Gerlach experiment. Subatomic particles such as electrons neutrons protons quarks and so on.
In Quantum mechanics the particle is described by a wavefunction xyzt It is related to the probability of finding an electron at time t in a volume dxdydz. The quantum mechanical model specifies the probability of finding an electron in the three-dimensional space around the nucleus and is based on solutions of the Schrödinger equation. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s.
In Quantum mechanics electrons are described by wave functions. This gives freedom of. This cloud of negative charge bonds the positively-charged nuclei of molecules together in a quasi-classical way since the nuclei are heavy and less quantum-mechanical in some sense.
It is called the conduction band because it is not full so electrons can move from one state to an empty one. We need a microscope or some other technology to detect the behavior of the smallest part of matter the atom. The principle quantum number is the energy level of an electron.
These are called the valence bands. This means that we shouldnt imagine electrons as single objects going. Quantum mechanics doesnt explain why electrons move around the nucleus it explains why they dont.
Used to explain the origin of spectral lines and to describe the electronic structure of the atom. Below are some examples of macroscopic quantum effects. Thinking about electrons as probabilistic matter waves using the de Broglie wavelength the Schrödinger equation and the Heisenberg uncertainty principle.
A true quantum theory would be completely free of. The objective reality of quantum mechanics is directly related to the understanding of the relation between c 2 and the mathematical properties of space and time. X yzt 2dxdydz or dxdydz or in 1D 1 1 2 dx x y z t dxdydz But since is a probability.
Quantum mechanics is a subfield of physics that describes the behavior of particles atoms electrons photons and almost everything in the molecular and submolecular realm. Quantum mechanics isn. However in order to describe electron diffraction you require quantum mechanics.
The way electrons dissociate from metals or move through semiconductors is described by solid state physics which draws heavily on quantum mechanics. Learn vocabulary terms and more with flashcards games and other study tools. But the Schrodinger Equation does not take Special Relativity into account.
Usually it is used only to describe the behavior of electrons because it is not accurate for faster-moving particles. You can calculate the solutions of the Schrödinger equation and if you take the potential of the nucleus into account you arrive at wavefunctions for the electrons in an atomThose. This is why quantum mechanics has to be mastered as part of learning theoretical chemistry.
The first quantum number describes the electron shell or energy level of an atom. An atomic orbital which is distinct from an orbit is a general region in an atom within which an electron is most probable to reside. Periods result from the filling of electron energy levels.
Our classical notions of fields and forces fail to capture the real effects of the quantum mechanical Universe demonstrating the need for them to be somehow quantized too. In essence it was largely derived using classical mechanics and then ending with Plancks energy quanta hypothesis. Often you can treat electrons in an atom as a cloud of charge whose charge density is proportional to the probability density for the electron.
You are asking something very broad which makes it hard to give a specific answer but generally yes. The unfilled shells make up the energy states in the conduction band. Quantum mechanics tells us that you cant precisely know both the position and velocity of an electron at the same time.

Quantum Numbers And Quantum Mechanical Model Diagram Quizlet

Quantum Mechanical Model Overview History Expii
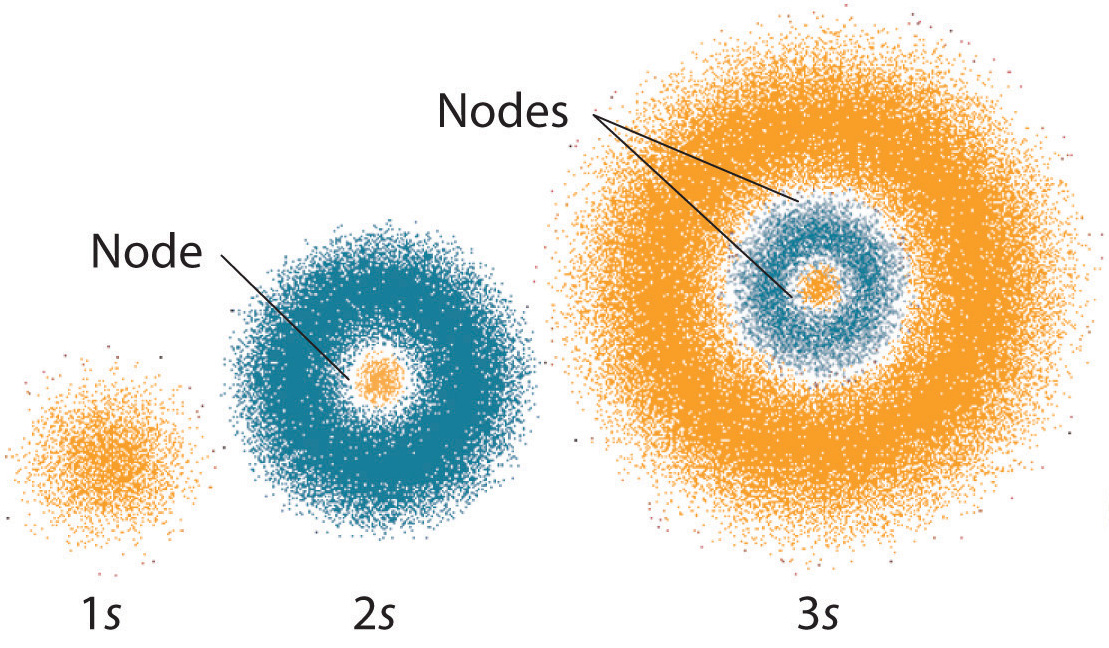
The Quantum Mechanical Model Of The Atom Article Khan Academy
Comments
Post a Comment